Accelerators for Sulfur Vulcanization of Rubbers
Vulcanization of rubber by sulfur alone is an extremely slow process and can take several hours at elevated temperature (140°C or more). This is problematic becasue long exposure to temperature and oxygen leads to oxidative degradation which, in turn, results in poor mechanical properties. It is also not very economical. To minimize rubber degradation and to speed-up the vulcanization process, accelerators are usually employed. An accelerator is defined as a compound that increases the speed of vulcanization and that enables vulcanization to proceed at lower temperature and with greater efficiency. Accelerator also decreases the amount of sulfur needed to cross-link the polydiene, which improves the aging properties of the vulcanized rubber. Some of the accelerators also function as sulfur donors and thus, allow vulcanization to proceed at lower sulfur content.
A large number of chemicals that belong to different chemical classes are known to accelerate rubber vulcanization, however, only about 50 accelerators are used on a commercial scale. Most of these belong to six classes, which are shown in the table below.
| Compound | Chemical Structure | Side Groups (R) |
|
Guanidine (Moderate) |
R = Phenyl, Toluoyl R = Alkyl |
|
|
Dithiocarbamate (Very fast) |
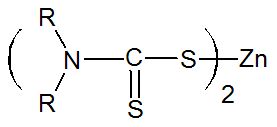 |
R = Phenyl, Toluoyl R = Alkyl |
|
Thiuram (Very fast) |
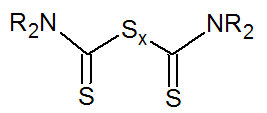 |
R = Alkyl |
|
2-Mercaptobenzothiazole |
|
|
|
Zinc-2-mercaptobenzothiazole |
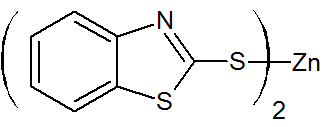 |
|
|
Thiourea (Very fast) |
|
R = Alkyl, Phenyl |
|
Benzothiazole Sulfenamide |
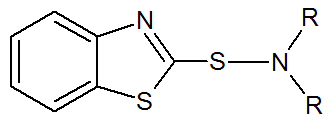 |
R = H, Alkyl R = Phenyl |
|
Isopropylxanthate |
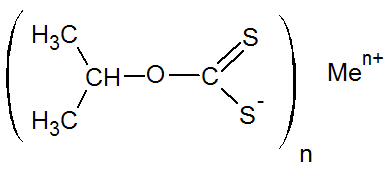 |
Men+ = Zn2+, Na+ |
The accelerators can be further classified as primary and secondary accelerators. The primary accelerators are typically used at 0.5 to 1.5 phr. The vulcanization speed of these system can range from slow to ultra fast depending on composition and type of accelerator. Common primary accelerators include thiazoles and sulfenamides whereas thioureas and dicarbamates can function as both primary and secondary accelerators. Very fast are thioureas and dicarbamates (see table above) and semi-fast are thiazoles whereas aldehydeamines and guanidines are rather slow. Some other curatives such as sulfenamides are fast curatives and cause a delay in the onset of vulcanization which is often desired in rubber processing because it increases the scorch time.2
Secondary accelerators are used to activate primary accelerators. They also greatly increase the speed of vulcanization. The dosage of secondary accelerators is typically only a fraction of that of the primary accelerator (about 0.05 to 0.5 phr). Common secondary accelerators include guanidines, thiurams, and dithiocarbamates. The later can also be used as a ultra-fast primary accelerator.
Both the cross-link density and the cure speed depend on the type and dosage of accelerator, or in other words, the number of sulfur atoms in the sulfur bridges, their average number per polymer and the reaction rate depend on the type and composition of the sulfur cure system.
Many of the sulfur-based vulcanization systems require activators. For example, dithiocarbamate and thiazole accelerators are activated with zinc oxide and stearic acid which also increase the speed of vulcanization.
An important factor in the vulcanization process is the cure temperature which also affects the crosslink density and structure. To minimize thermal and oxidative degradation vulcanization should be done at the lowest possible temperature. However, to increase productivity, higher cure temperatures are often chosen.
Notes
-
Scorch is the premature cross-linking of the rubber before the final molding is achieved.
It usually results in scrap. -
The scorch time is the time during which a rubber can be processed and molded..